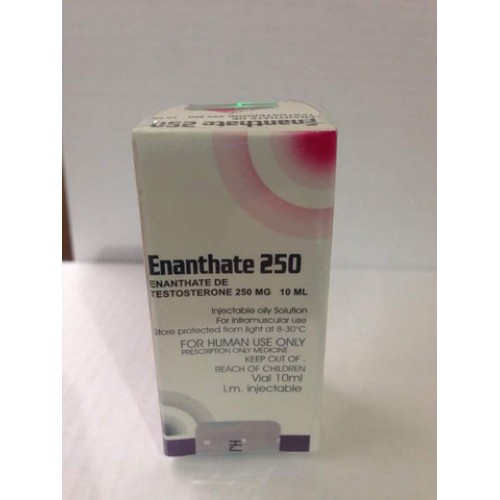
Description
PRESENTATION: Vial of 10 ml.
EACH ML CONTAINS: 250 Mg. of Testosterone Enanthate.
Laboratory: Karlskoga Labs.
APPLICATION: Intramuscular injection.
Testosterone Enanthate is a perfect steroid for all levels of use. This is the perfect anabolic steroid for a beginner and will be equally effective for advanced athletes. It will carry with it possible side effects, but you will also find that they are very easy to control. This really shouldn’t be surprising, after all, although synthetic it is simply testosterone, a hormone that the human body is not only well accustomed to, but is essential for our health and well-being. Functions and Traits: Testosterone Enanthate is a single large ester based testosterone compound. This is a pure synthetic testosterone hormone that has a carboxylic acid ester bonded into Enanthate (enanthoic acid). The ester itself is attached to the hormone at the 17beta hydroxyl group. With the placement of the enanthate ester, this allows for control of hormone release. Once injected, testosterone is not activated until the ester begins to break off from the hormone. Total shedding does not happen all at once, but it allows for a slow and steady release of the active hormone in the body. From here the hormone will continually break apart and dissipate throughout the body. Per its time frame, Testosterone Enanthate carries a half-life of approximately 8 days, which will allow for as little as one injection every 2 weeks in a therapeutic setting. However, applying it every 7 to 10 days will be much more effective in maintaining stability.
THIS PRODUCT IS NOT RECOMMENDED FOR PEOPLE WITH CHRONIC DISEASES, THE USE OF IT IS THE RESPONSIBILITY OF THE PERSON WHO CONSUMES IT, CONSULT YOUR DOCTOR.


Reviews
There are no reviews yet.